Some very recent studies also argue that AAs could be processed in the cloud medium by the biological activity Bianco et al 2019. Is dissolution endothermic or exothermic.
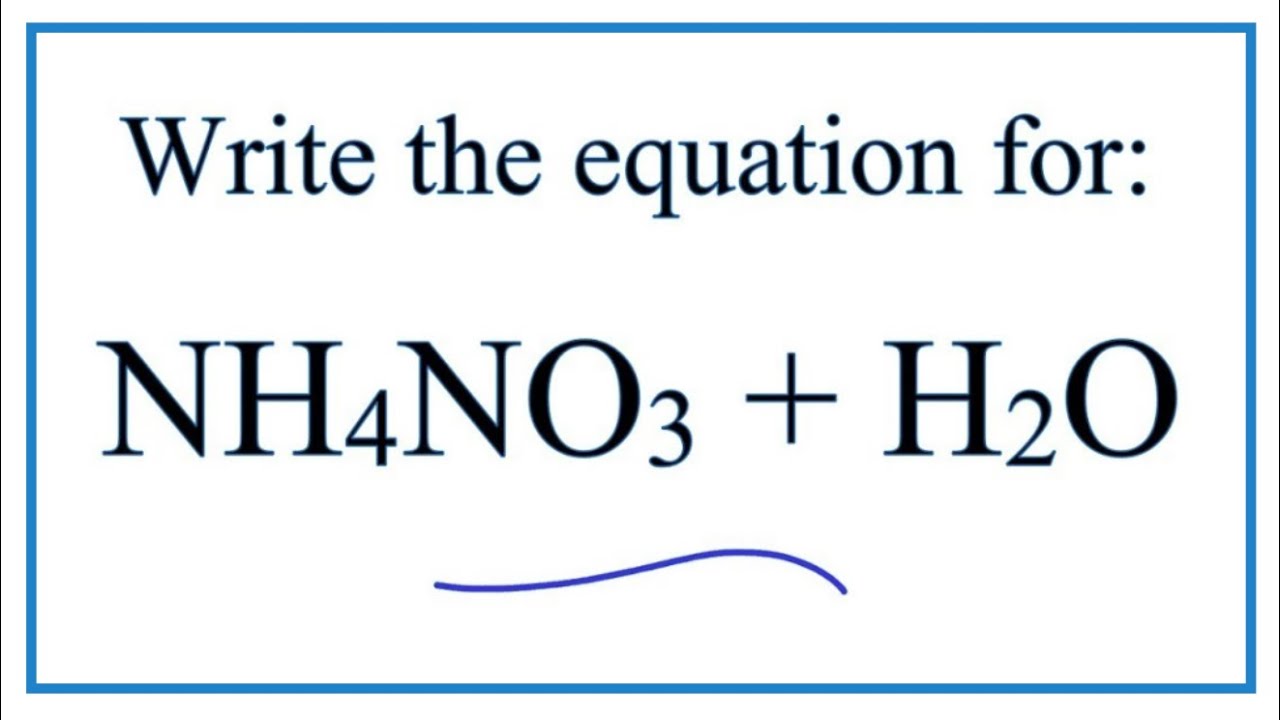
Equation For Ammonium Nitrate Dissolving In Water Nh4no3 H2o Youtube
The subscript aq in the equation signifies that the sucrose molecules are solutes and are therefore individually dispersed.

. 1 point a The reactants would appear at a lower energy state than the products. The dissolution of calcium hydroxide in water is also an exothermic process ΔH 0 and obeys the van t Hoff equation and Le Chateliers principle. Electrocatalytic recycling of waste nitrate NO3 to valuable ammonia NH3 at ambient conditions is a green and appealing alternative to.
AA concentration in cloud water results from the dissolution of the soluble fraction of the aerosol particles acting as CCN and IN. Because more energy. This is an example of a phenomenon known as the common ion effect which is a consequence of the law of mass action that may be explained using Le ChÂteliers principle.
B The reactants would appear at a higher energy state than the. Water is a reactant of our original reaction and when we added it the concentration of the cobalt complex ion on the right side of the equation increased. A lowering of temperature favors the removal of dissolution heat from the system and thus favors dissolution of CaOH 2.
Such as ammonium nitrate dissolve in wateran endothermic process. Fertilizer injectors are devices used to apply water-soluble fertilizers pesticides plant growth regulators wetting agents and mineral acids during crop production. A solution forms when two or more substances combine.
As the ammonium nitrate dissolves heat energy is absorbed from the environment causing the surrounding environment to feel cold. NH4NO3s heat NH4aq NO3-aq Which statement best describes what an energy graph of this reaction would look like. Despite the advantages many growers have had at least one experience with a compromised damaged or even ruined crop where the.
Thus 1 mol of ammonium dichromate formula units dissolves in water to produce 1 mol of Cr 2 O 7 2 anions and 2 mol of NH 4 cations see Figure PageIndex4. Write the ionic equation for the dissolution and the solubility product expression for each of the following slightly soluble ionic compounds. The dissolution of ammonium nitrate in water is endothermic ΔH soln 257 kJmol whereas the dissolution of calcium chloride is exothermic ΔH soln 682 kJmol yet Figure 139 Solubilities of Several Inorganic and Organic Solids in Water as a Function of Temperature shows that the solubility of both compounds increases sharply with increasing temperature.
So portlandite solubility increases at low temperature. The process of dissolving is exothermic when more energy is released when water molecules bond to the solute than is used to pull the solute apart. The principle of this type of analysis is that once an ions mass has been determined as a unique compound that known measurement can then be used to determine the same analytes mass in a mixture as long as.
What is the ammonium nitrate concentration in the resulting solution. Dissolution of 1 mol of an Ionic Compound. The silver ions react with the chloride and make silver chloride precipitates which look like white solids.
Titration Questions and Answers. Compared with pure water the solubility of an ionic compound is less in aqueous solutions containing a common ion one also produced by dissolution of the ionic compound. They are a vital part of modern greenhouse or nursery operations.
Access the answers to hundreds of Titration questions that are explained in a way thats easy for you to understand. Watch this brief video illustrating endothermic and exothermic dissolution processes. Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte the ion being analyzed based on its mass.
Water weakens urines concentration significantly reducing its visibility for drug substances. For instance the biodegradation of AAs was demonstrated to occur in rainwater Xu et al 2020 and in. A 500 mL sample of 0436 M NH4NO3 is diluted with water to a total volume of 2500 mL.
Dec 14 2021 Meths primary ingredients ephedrine and pseudoephedrine come from over-the-counter cold medications and weight loss products. Dec 10 2019. Get help with your Titration homework.
Key Concepts and Summary. Jul 16 2019 In 2017 526 meth-related fatal overdoses were reported in Ohio more than twice the number from 2016 and 2. A AgI silver iodide a solid with antiseptic properties b CaCO 3 calcium carbonate the active ingredient in many over-the-counter chewable antacids c MgOH 2 magnesium hydroxide the active ingredient in Milk of Magnesia d MgNH 4PO.
6-The dissolution of ammonium nitrate in water is given by the following equation. 5 Silver nitrate readily reacts with the chloride ions available in the solution.

Equation For Ammonium Nitrate Dissolving In Water Nh4no3 H2o Youtube

Solved 9 18 Points Here Is The Chemical Equation For The Chegg Com
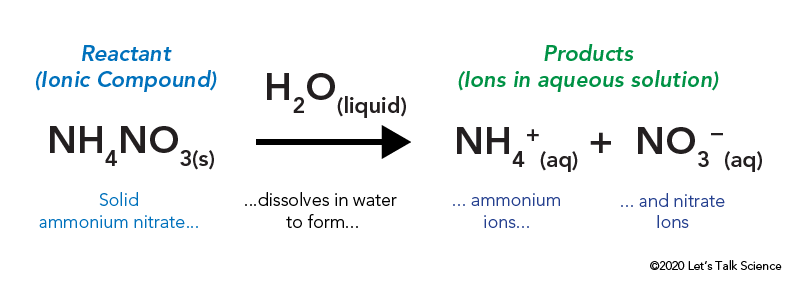
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
0 Comments